|
All atoms are created by stars (very high heat/pressure source) that go supernova.
It is very possible that an atom in your right hand and one in your left hand comes from different stars.
All atoms are 99.9999% empty space.
Imagine a large circular football stadium.
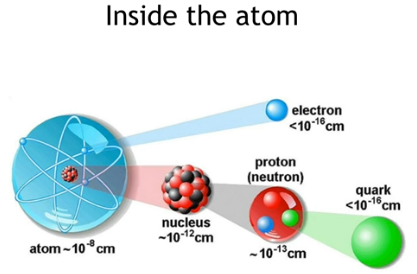
An atom consists of three components.

The Mass Number of an element is the number of neutrons and protons in the nucleus. The Atomic Number is the number of protons in the nucleus. The number of neutrons is the Mass Number minus the Atomic Number. The number of electrons is equal to the number of protons or the Atomic Number.
Electrons create spheres (electron clouds) around the nucleus.
Valency is associated with the number of outer electrons that cause chemical bonding of atoms. Isotopes: atoms with varying neutrons Ions: atoms with varying electrons I recently saw a new field of research called Atomic Engineering. This is lower level work from Molecular Engineering.
Gold is an atom that tends to associate more strongly with other gold atoms.
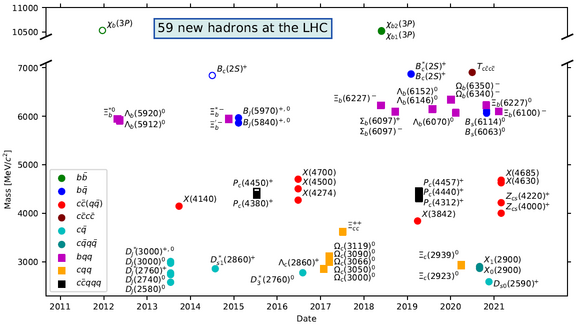
It was in December, 1938 that we discovered fission (splitting of atoms). An element is a substance that is made up of atoms that are alike and that cannot be broken down into any other substance. Ninety nine (99) percent of a person consists of just six elements. (hydrogen, carbon, nitrogen, phosphorus, sulfur, and oxygen) A molecule is a group of two or more atoms that are bonded together. Pubchem: Periodic Table of Elements Covalent bonds and molecules A covalent bond is formed when two atoms share electron pairs. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two positively charged atomic nuclei and the shared, negatively charged electrons between them. Unlike covalent bonds, in which electron pairs are shared between atoms, an ionic bond is formed when two oppositely charged ions attract one another. If an atom gains or loses electrons, the balance between protons and electrons is upset, and the atom becomes an ion—a species with a net charge.
SubAtomics Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, making up the nucleus. Along with neutrons, protons make up the nucleus, held together by the strong force. The proton is a baryon and is considered to be composed of two up quarks and one down quark. The charge on the proton and electron are exactly the same size but opposite. Along with protons, neutrons make up the nucleus, held together by the strong force. The neutron is a baryon and is considered to be composed of two down quarks and one up quark. A free neutron will decay with a half-life of about 10.3 minutes but it is stable if combined into a nucleus. The number of protons in an element's nucleus is called the atomic number.
Protons are made of two Up and one Down quark. The neutron is made of two Down and one Up quark. The Up quarks have a 2/3 positive charge and the Down has a 1/3 negative charge. Protons and neutrons each contain three quarks. A proton is composed of two 'Up' quarks and one 'Down' quark while neutrons are composed of one 'Up' quark and two 'Down' quarks. Nucleons and Quarks Neutrons and protons are made up of quarks, which are held together by Gluons. Since the number of protons in an atom does not change, fewer or extra electrons can create a special atom called an ion. Cations have fewer electrons and have a positive charge. Anions have extra electrons that create a negative charge. When two or more quarks are held together by the strong nuclear force, the particle formed is called a hadron.
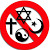
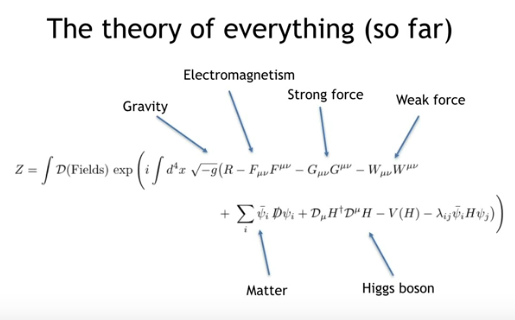
Standard Model of Particle Physics:
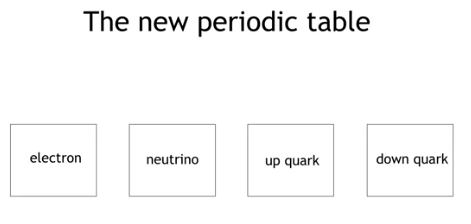
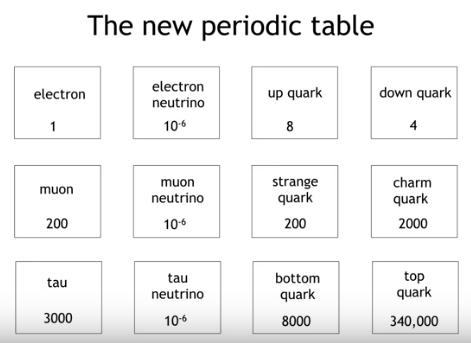
There is a list of all subatomic particles known to date, but the list is constantly evolving as new particles are discovered and existing ones are further studied. Quarks: Quarks are elementary particles that make up protons and neutrons, which are the building blocks of atomic nuclei. There are six types of quarks: up, down, charm, strange, top, and bottom. Leptons: Leptons are another type of elementary particle that are not composed of smaller particles. There are six types of leptons: electron, muon, tau, and their corresponding neutrinos. Gauge bosons: Gauge bosons are particles that carry the fundamental forces of nature. There are four known gauge bosons: photon, W and Z bosons, and gluons. Higgs boson: The Higgs boson is a particle that is associated with the Higgs field, which is responsible for giving particles mass. Mesons: Mesons are particles made up of a quark and an anti-quark. They are often produced in high-energy collisions. Baryons: Baryons are particles made up of three quarks. Protons and neutrons are examples of baryons. Antimatter particles: Antimatter particles are the opposite of normal matter particles. They have the same mass as their corresponding matter particles, but opposite charges.
Although, by volume, an atom is mostly empty space, dominated by the electron cloud, the dense atomic nucleus, responsible for only 1 part in 10^15 of an atom’s volume, contains ~99.95% of an atom’s mass. Reactions between internal components of a nucleus can be more precise and occur on shorter timescales, as well as at different energies, than transitions restricted to an atom’s electrons. |
|||||||||||||||
Send comments to:
 hjw2001@gmail.com
hjw2001@gmail.com
|